Article Text
Statistics from Altmetric.com
Introduction
Cardiovascular diseases are the leading causes of death globally and are increasing.1 Risk factors can be effectively treated, but more people need to be treated more aggressively for the high-risk strategy to be effective.2 Proposed solutions include polypills3 and risk-based approaches4 5, the latter recognising the merits of basing treatment decisions for the prevention of cardiovascular disease on a person’s predicted absolute risk of disease, rather than on the level of a single risk factor.6 7 Both solutions need the answer to a specific, but hitherto overlooked question: are there any synergistic effects between the preventive drug treatments?
When a high-risk person is identified as a candidate for primary or secondary prevention against cardiovascular disease, the potential drug regimen is likely to include blood pressure-lowering drugs and statins. Combining these two treatments may result in anything from less than an additive effect to more than a multiplicative effect on cardiovascular disease risk. Experimental studies have suggested positive8 9 as well as negative10 synergistic effects between blood pressure-lowering drugs and statins.
Several factorial trials with these two treatments have been made in humans, but as a whole, their interaction is unknown. With the recent addition to that literature,11 the evidence base may now be sufficient to answer the question. We hypothesised that the combined effects of blood pressure-lowering drugs and statins are multiplicative. In order to test the hypothesis, we performed a systematic review of randomised factorial trials of the effects of blood pressure-lowering drugs and statins on cardiovascular outcomes in primary or secondary prevention.
Methods
Data sources and searches
Using a pre-specified systematic review protocol, we performed data searches in MEDLINE, SCOPUS, Cochrane Central Register of Controlled Trials and ClinicalTrials.gov, and Web of Knowledge conference abstracts, and limited the search from earliest available to December 2017. The MEDLINE search strategy was: (1) randomised controlled trial (pt); (2) controlled clinical trial (pt); (3) randomised (tiab); (4) clinical trial (all); (5) randomly (tiab); (6) trial (ti); (7) placebo (ti); (8) 1 or 2 or 3 or 4 or 5 or 6 or 7; (9) hypolipidaemic agents (mesh); (10) hydroxymethylglutaryl-CoA reductase inhibitors (mesh); (11) statin (tw); (12) 9 or 10 or 11; (13) antihypertensive agents (mesh); (14) cardiovascular (all); (15) cardiovascular diseases (majr); (16) 14 or 15; (17) 8 and 12 and 13 and 16. Similar search strategies were applied to the other sources. Reference lists of relevant publications were hand searched.
Study selection
Studies were included in the systematic review if they were factorial and had at least two randomised interventions, had a total duration of at least 100 patient-years, involved patients aged ≥18 years, had at least one statin and one blood pressure-lowering drug as active treatment, had placebo or a less intensive blood pressure-lowering drug regimen as control treatment, and reported clinical cardiovascular events or mortality as outcomes. Trials were excluded if they did not report clinical outcomes or if the intervention strategies were unclear. Due to scarcity of data, we included studies with different intensities of antihypertensive treatment in the blood pressure factor. We defined treatment groups in these studies as more versus less intensive antihypertensive treatment based on achieved blood-pressure differences on the group level. In case of several useful publications from the same study, the one with the longest duration of follow-up was used. Two independent reviewers screened all abstracts for eligibility, reviewed relevant articles in full text, included relevant articles in the systematic review and, if applicable, extracted data for the meta-analysis.
Data extraction and bias assessments
Data were extracted from the articles using a prespecified spreadsheet. In case of unclear reporting of results, we contacted the authors asking for supplementary information. Data items extracted included study identification variables, treatments, numbers of patients, age, sex, baseline variables (body mass index, smoking, known hypertension, known dyslipidaemia, previous cardiovascular disease, previous myocardial infarction, type 2 diabetes, systolic and diastolic blood pressures, total, high-density lipoprotein cholesterol and low-density lipoprotein cholesterol; described in the online supplementary table 1) and outcome variables (major adverse cardiovascular events (MACE, as defined by the studies, mainly including myocardial infarction, stroke and cardiovascular death, optionally also heart failure), MACE-plus (expanded MACE classifications, as defined by the studies, usually adding unstable angina and coronary revascularisation), myocardial infarction, stroke, cardiovascular death, and total death; described in the online supplementary table 2). Quality of the included trials was gauged by using the Cochrane Collaboration’s risk-of-bias tool. Publication bias was assessed using funnel plots and Egger’s tests.
Supplementary file 1
Data synthesis and analysis
Baseline data were summarised using inverse variance weights. Treatment effects were visualised in forest plots of tabular trial data, using fixed-effects inverse variance-weighted meta-analysis models to illustrate heterogeneity as the I2 statistic.
In order to investigate synergistic effects, we used a one-step individual patient data meta-analysis approach12 with a two-level mixed-effects logistic regression model with patient as the unit of analysis and trial modelled on a second level with random intercept. Models with the addition of random coefficients for treatments did not have a better fit than models with only a random intercept and were not further pursued. This is the recommended one-stage model for assessing within-trial interactions.13 14 Because the aggregate data used in this study are all dichotomous variables, a complete representation of the individual participant data for the primary analyses could be achieved.14
Because there is no generally accepted statistical definition of pharmacological synergism, we tested for interactions in two ways: as departure from additivity and as departure from multiplicativity. Departure from additivity was investigated as the relative excess risk due to interaction (RERI), the attributable proportion due to interaction (AP) and as the synergy index (S),15 in the mixed-effects models described above. In the absence of interaction on the additive scale, RERI and AP are equal to 0 and S is equal to 1. Departure from multiplicativity was examined using a within-trial product of the variables for statins and blood pressure-lowering drugs, added to the otherwise same mixed-effects models. In the absence of interaction on the multiplicative scale, the OR for that product is equal to 1.
We used two-sided 95% CIs for all hypothesis tests. Stata V.14.2 was used for all analyses.
Patient involvement
No patients were involved in the design, conduction, interpretation or reporting of the analyses.
Results
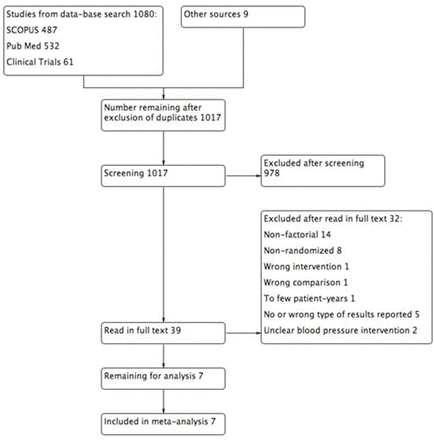
Out of 1017 studies screened, 39 were read in full text and 711 16–21 were eventually included in the overview (figure 1). Using funnel plots (online supplementary figure 1), no evidence of publication bias was observed (all Egger’s test P>0.12). Risk of bias within studies was generally low (online supplementary figures 1 and 2).
Flow chart of literature review.
The included trials contributed a total of 27 020 patients. Baseline characteristics are described by the study group in table 1, and by trial in the online supplementary table 1. The trials were heterogeneous in terms of target populations, with some recruiting from the general population and some only among patients with established cardiovascular disease or organ damage. Baseline characteristics were well balanced between the randomised groups.
Baseline characteristics
The trials contributed 1560 MACE-plus events, 857 MACE events, 409 strokes, 144 myocardial infarctions, 725 deaths and 348 cardiovascular deaths. Relative risk reductions with blood pressure-lowering drugs/more intense blood pressure-lowering regimen and statins are presented per trial in the online supplementary figure 4.
The relative risk reduction with blood pressure-lowering drugs/more intense blood pressure-lowering regimen was not materially different in subgroups randomised to statins or placebo (figure 2). Likewise, the relative risk reduction with statins was not substantially different in subgroups randomised to blood pressure-lowering drugs/more intense blood pressure-lowering regimen or placebo/less intense regimen (figure 3).
Effects of blood pressure-lowering treatment by subgroups of statin treatment. BPRx, blood pressure-lowering treatment; MACE, major adverse cardiovascular events; RR, risk ratio.
Effects of statin treatment by subgroups of blood pressure-lowering treatment. BPRx, blood pressure-lowering treatment; MACE, major adverse cardiovascular events; RR, risk ratio.
Interaction analyses did not reveal any departures from either additivity or multiplicativity (table 2). The analysis cannot exclude the possibility of a small synergistic effect between the randomised treatments on major cardiovascular events, but it is not statistically significant given the current evidence base.
Departures from additivity and multiplicativity of effects
Heterogeneity was low overall; hence, subgroup analyses were not called for. Results were largely driven by findings in two large trials (online supplementary figure 4).
Discussion
In this systematic review of randomised factorial trials, the joint relative effects of blood pressure-lowering drugs and statins on major cardiovascular events appeared multiplicative. The analysis cannot exclude the possibility of a slightly more than multiplicative effect between the treatments on major cardiovascular events, but it is unlikely that the combined effect is less than multiplicative.
There is some experimental evidence regarding pharmacological synergism between blood pressure-lowering drugs and statins, with a few studies proposing potentiating synergistic effects8 9 and one suggesting diminished combined effects.10 In humans, observations in the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT)20 supported synergistic effects, but the recent Heart Outcomes Prevention Evaluation-3 (HOPE-3) study did not.11 While differences between the two studies may be explained by the different drug regimens (candesartan/hydrochlorothiazide vs placebo and rosuvastatin vs placebo in HOPE-3 and amlodipine/perindopril vs atenolol/bendroflumethiazide and atorvastatin vs placebo in ASCOT) or different populations (higher risk sample in ASCOT with markedly higher blood pressure), they may also be due to chance (the full-factorial combinations underpowered compared with the primary comparisons in the original studies). With the latest addition to the evidence base, we assumed it large enough now to answer the question.
The question is of substantial clinical importance. With the increasing recognition of similar relative effects of statins and blood pressure-lowering drugs across the whole spectra of cholesterol and blood pressure,6 7 and consequent merits of risk-based treatment decisions, the clinician needs to know how to treat a patient identified to be at high risk. Any potentiating synergistic effects should steer the treatment choice towards introducing both statins and blood pressure-lowering drugs in high-risk people in primary prevention, irrespectively of cholesterol and blood pressure levels. Likewise, any potentiating synergistic effects should also argue for fixed-combination polypills.3 In contrast, a less than additive combined effect should likely focus more on treating single risk factors aggressively. The present study shows that combination treatment with statins and blood pressure-lowering drugs on average gives a combination of at least the anticipated relative risk reductions of each of the treatments.
Weaknesses of this study include the low power for analyses of some outcomes and heterogeneous treatment regimens in the included studies, which on the other hand all are representatives of drug classes available to answer the research question. Further, heterogeneity in results was fairly low in spite of the different treatments. Strengths of this study include the large sample relevant for prevention situations in primary care, stringent systematic review methods including transparent analysis of several biases, and analyses of interaction on both the additive and multiplicative scales. It should be noted that definitions and analytical operationalisations of synergy are debated.22 Using analogies to pharmacological analyses of drug combinations, the analyses of the present study reflect effect-based rather than dose–effect-based strategies, and most closely reflect the Bliss Independence model. Synergy can be assessed as departure from additivity on an absolute risk scale, and as departure from multiplicativity on a relative risk scale. A strength of the analysis framework in this study is that incorporates both analyses.22
In sum, the combined relative effects of blood pressure-lowering drugs and statins on major cardiovascular events were multiplicative in this systematic review of factorial trials. The possibility of a slightly more than multiplicative effect between the treatments could not be excluded and future factorial trials may add relevant evidence.
References
Footnotes
Contributors MW performed literature review, collected the data, interpreted the data and contributed to the writing of the article. GG performed literature review, collected the data and interpreted the data. JS designed the study, handled funding, supervision, statistical analysis, interpreted the data and wrote the article. JS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests JS reports being on the advisory board for Itrim, outside of the study. GG and MW report no competing interests.
Provenance and peer review Not commissioned; externally peer reviewed.